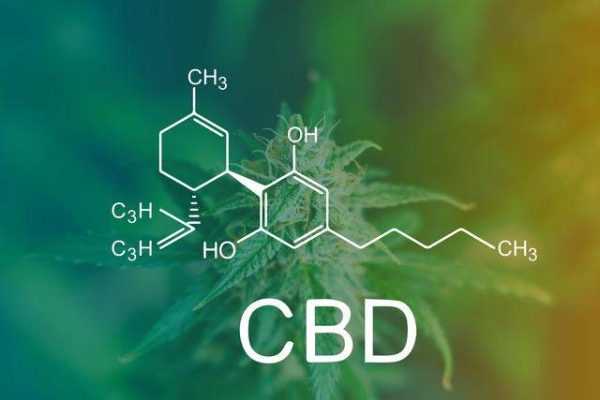
A piece in Cannabis Wire offers some insight into the FDA's decision to reject CBD's use as a dietary supplement. Essentially, the agency doesn't want to approve CBD as a additive to mainstream products when it's also the active ingredient...
This content is FREE for registered users.
To view this content you need to register as a Weedweek member.
If you are already a member please login below.
pro
Lawsuit alleges SoCal city extorted licensees
As the Department of Cannabis Control (DCC) tries to expand the number of cities and counties with cannabis businesses, an April lawsuit details how local officials in one SoCal city allegedly blocked a group of cannabis businesses from opening for...
sponsored
The Best 420 Deals in Los Angeles for 2024
Are you looking for the best cannabis discounts and events for April.20.2024 in Los Angeles? Today we’re putting a spotlight on the LAX Cannabis Club dispensary, which is conveniently located a short drive from the LAX airport. Therefore, it's the...
pro
What should Calif. do about hemp?
Last week, the California State Assembly’s Business and Professions Committee unanimously cleared a bill that would require intoxicating, hemp-derived products to be regulated much like licensed cannabis and sold in dispensaries. The 18-0 vote indicates lawmaker interest in addressing an...